Recently, there have been conflicting reports regarding the
use, safety and efficacy of the diaphragm pacing system (DPS) developed by
Synapse Biomedical in ALS patients. These reports included a peer-reviewed
publication of a study called DiPALS
conducted in the United Kingdom and a press release stating that results from Synapse’s
own clinical trial results will be presented at the annual ALS/MND Research
Symposium to be held this year in Orlando, Florida. The DiPALS study found
those whom had the DPS implanted were more likely to succumb to ALS than those
whom used non-invasive ventilation alone whereas the Synapse press release stated
that their results were much more positive. Since only the DiPALS study results
have been published, it is a challenge to compare the two studies appropriately
at this time.
 What is DPS?
What is DPS?
While the exact causes of all ALS cases are not known, it is
generally accepted that as the disease progresses, the ability of an individual
to breathe entirely on their own will be lost. Breathing is controlled in part
by the diaphragm; an important muscle connected to the lungs. However, in ALS,
a person’s motor neurons disconnect from their muscles, which causes those
muscles to waste away. As with other muscles, the diaphragm atrophies as a
person’s ALS progresses. Some people with ALS will choose to use non-invasive
ventilation (NIV) technologies, such as a Bi-Pap, to assist in their
respiration. Later on in the disease, some people diagnosed with ALS will
choose to connect to a permanent ventilator.
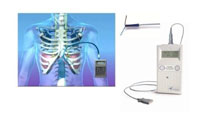
The NeuRx Diaphragm
Pacing System (DPS, pictured) system was developed in 2005 by surgeon Raymond Onders
and colleagues at Case Western Reserve University and University Hospitals of
Cleveland. The idea for the device was to augment the electrical signaling of
the diaphragm necessary for respiration which was lost as ALS progresses and
the natural connections between the diaphragm and the nervous system
deteriorates. The device consists of five electrodes and an external pulse
generator. When activated, the generator sends small electrical signals to the
electrodes connected to the diaphragm muscle, causing it to become stimulated.
By stimulating the diaphragm, the device is designed to aid in respiration, not
a permanent replacement for the diaphragm itself.
In 2011, DPS was provided a Humanitarian
Use Exemption by the FDA, which means that its effectiveness has not yet
been demonstrated. However, on-going clinical research continues on the device,
including the research in the two studies recently reporting out results.
What occurred in
the Synapse Biomedical Post Approval Study (PAS)?
Following the Humanitarian Device Exemption from the FDA,
Synapse Biomedical launched a post approval study (PAS) as is required by the
FDA. That study occurred in 11 different sites in the United States online, and
it completed its enrollment of 60 PALS in August 2014. No data outside of that which was provided in
the press release has been made available. However, according to the press
release, the results of the PAS were similar to the original study – with
the median survival of PALS being 20.9 months post-implant. The original study included 106 PALS and was
conducted in 9 centers and had a median survival in PALS of 19.7 months
post-implant. Dr. Onders will provide a talk at the Symposium
on the original study, whereas Dr. Robert Miller, principal investigator of the
PAS, will provide a presentation on the new data from Synapse.
According to Synapse CEO, they are confident that their
device is safe and effective when used in patients that are “carefully selected
and screened according to proper criteria.”
While it is not entirely clear if this is a suggestion that others have
used the device without properly selecting or screening PALS, we expect that
these criteria will be discussed at length during the talks from Drs. Miller
and Onders later this year.

What occurred in
the DiPALS study?
This was an independent
study conducted at 7 sites in the United Kingdom which enrolled 74 PALS
between 2011 and 2013. The final data from the study was collected in December
of last year; the paper was published this July. The study aimed to determine a
number of different things, but most generally whether or not the use of DPS
together with NIV impacted the survival of PALS. Those not selected for the
implantation group acted as the control group receiving NIV only when
determined necessary as part of the standard of care of PALS. In total, 32
people in the study underwent DPS implantation.
All studies in the UK are monitored by independent boards of
overseers, which in this case with the Data Monitoring and Ethics Committee.
That committee determined that the cohort of PALS who received the implantation
were succumbing to ALS quicker than those who were on NIV alone. As result, the
committee advised the discontinuation of pacing in all patients and the trial
stopped. The investigators in the study state that DPS should not be a routine treatment in PALS with
respiratory failure.
 In comparing their findings to the original work from
Synapse which led to the DPS receiving Humanitarian Use Exemption from the FDA,
the DiPALS investigators, led by principal investigator Dr. Chris McDermott (pictured),
believe that their study was more representative of the general ALS population
than the original work. For example, the DiPALS team suggests that the earlier
study enrolled more slow progressing PALS in their study because they followed
PALS for a period of 3 months before determining whether or not to enroll them,
whereas in the DiPALS study they enrolled all PALS with the intent to treat,
and randomized regardless of an individual’s progression rate. The DiPALS team
leaves open the possibility that a subgroup of ALS patients may be able to be
identified and benefit from DPS, but that a “nothing to lose” approach to
treating ALS may be harmful to patients.
In comparing their findings to the original work from
Synapse which led to the DPS receiving Humanitarian Use Exemption from the FDA,
the DiPALS investigators, led by principal investigator Dr. Chris McDermott (pictured),
believe that their study was more representative of the general ALS population
than the original work. For example, the DiPALS team suggests that the earlier
study enrolled more slow progressing PALS in their study because they followed
PALS for a period of 3 months before determining whether or not to enroll them,
whereas in the DiPALS study they enrolled all PALS with the intent to treat,
and randomized regardless of an individual’s progression rate. The DiPALS team
leaves open the possibility that a subgroup of ALS patients may be able to be
identified and benefit from DPS, but that a “nothing to lose” approach to
treating ALS may be harmful to patients.
Bottom Line
This is a confusing topic for anyone in the ALS community
whether they are a patient, family member, care provider, neurologist or allied
health professional. The DPS story has been an evolving one for more than a
decade and there are great differences of opinion between clinics on its
potential efficacy and appropriate use. One of the things to note about these
recent reports is that they were done in two different countries where the
standard of care and practice in treating ALS patients can differ, so it is not
as easy as comparing them directly to each other.
Without all of the data available for scrutiny from the new
PAS study from Dr. Miller, it is challenging at this time to compare and
contrast beyond the top line statements made. We will look forward to seeing
that data in Orlando later this year. Since the DiPALS data has been published,
we can comment further. It was a well designed study and seems to have been
well-controlled as well. There are some questions left open, however, within
any of the studies mentioned in this piece, and we look forward to
investigating those as well (i.e. were there enrollment site specific adverse
events, was there a relationship to FVC at implantation and median survival,
was there a co-morbidity relationship in those whom had the device implanted
and later removed, etc). Many of the unanswered questions are whether or not
there is a specific “type” of ALS patient or correct “time” in the disease
course to implant individual patients.
Through it all, PALS considering DPS should consult closely
with their medical team regarding whether or not it may be an appropriate
option for them. The device has still been granted a Humanitarian Use Exemption
from the FDA and individuals must obtain a prescription from a doctor to get
it. There are also strict limits set by the FDA on who can receive the device
under the Exemption, most notably perhaps to ALS patients is that a person must
have a FVC of greater than 45% at the time of implantation, and even then, if
the diaphragm is found to not respond to stimulation during surgery or if there
are other barriers found, the device will not be implanted. We encourage PALS
and CALS to utilize online patient led websites, such as PatientsLikeMe and our ALS Forum, to interact with PALS whom
have been implanted to learn more about those individual’s results.
Helpful Links:
DISCLOSURE: The ALS Therapy Development Institute invited Dr.
Onders to present his data on the device’s use in 2007 at our annual Leadership Summit. As with
all Summit speakers, Dr. Onders’ travel costs were paid for by the Institute.
As is allowed, we will report findings from the Synapse poster in Orlando via
Twitter (@ALSTDI) as well as in a
formal report to be posted to this website.
Image Sources: Dr. McDermott's photo is from the MNDA Blog Post, the NeuRX image from Synapse's press release and the Nature Article image is of the first page of the PDF of that article.