The 27th Annual International Symposium on
ALS/MND took place in Dublin, Ireland from December 7th to the 9th. This meeting is the largest science
conference in the world for ALS, held annually in different countries. This
year saw a record attendance of more than 1100, including scientists from more
than 30 different countries. The three day meeting typically includes two
simultaneous tracks; one on basic science such as genetics and biology and the
other on clinical science, covering care topics such as breathing,
communication and cognition as well as updates on clinical trials conducted on
potential treatments for ALS. In addition, alternative tracks were included
this year on biomarkers, neuroimaging, mouse models and quality of life.
 A total of 24 sessions occurred, most with 6-10
presentations each lasting 15-30 minutes. The first two days of the symposium
were followed by poster presentation sessions, during which an additional 200
sets of scientific findings were presented. The annual symposium also plays
host to major annual meetings of important working groups from the WFN, ENCALS,
NEALS, WALS and others. Many pharmaceutical and medical device companies were
also in attendance and hold break-out sessions.
A total of 24 sessions occurred, most with 6-10
presentations each lasting 15-30 minutes. The first two days of the symposium
were followed by poster presentation sessions, during which an additional 200
sets of scientific findings were presented. The annual symposium also plays
host to major annual meetings of important working groups from the WFN, ENCALS,
NEALS, WALS and others. Many pharmaceutical and medical device companies were
also in attendance and hold break-out sessions.
The symposium is organized annually by the Motor Neurone
Disease Association (MNDA) of England, Wales and Northern Ireland in
collaboration with a local host organization, which this year was the Irish
Motor Neurone Disease Association (IMNDA). These organizations collaborate to
form a program committee that is charged with planning the program for the
annual meeting and reviewing some 600 abstract submissions.
The following recap includes a few highlights, especially
from the clinical science sessions of the symposium. A public webinar was also
held by the author of this report, recapping these and selected other findings
reported. That webinar is available for review online by clicking here. It is
estimated that more than 100,000 people with ALS contributed either a sample or
their time to the basic and clinical research reported on during this year’s
symposium.
Founder of ALS TDI
Receives Humanitarian Award
Jamie Heywood and his brother Benjamin Heywood were jointly
presented the Humanitarian Award from the International Alliance of ALS/MND
Organizations during the joint opening session of the research symposium. Carol
Birks, chairperson of the Alliance and CEO of MND Australia, made the
presentation. Neither Jamie nor Ben were present, but provided a video
acceptance which you can view here. Steve Perrin, Ph.D. CEO of the ALS Therapy
Development Institute, and Paul Wicks, Ph.D., Vice President of Innovation for
PatientsLikeMe accepted the award on behalf of the Heywood brothers.
 Eating, Breathing
and Exercise Impact ALS
Eating, Breathing
and Exercise Impact ALS
More than one of the 24 sessions during the three-day
symposium touched upon these topics from countries across the globe, including
Australia, The Netherlands and the United States of America.
Rebekah Ahmed, MBBS, of Neuroscience Research Australia, found
that those with a higher body mass index (BMI) are more likely to also have
some form of frontotemporal dementia (FTD) together with their ALS (ALS-FTD).
Ahmed conducted a study of 143 people, including those with significant
cognitive and behavioral variants of ALS, ALS-FTD and FTD. Specifically, the study cohort contained 41
cases of ALS that either didn’t display any or sufficient signs of cognitive
impairment, 21 cases of ALS-FTD, 56 cases of behavioral variant FTD and 25
control cases (no signs of ALS or FTD).
Generally speaking, Ahmed’s data showed that those with
ALS-FTD were more likely to show signs of abnormal eating associated with a
higher BMI. Her research suggested that those with ALS-FTD and a higher BMI
survived longer than others studied in her research.
As ALS progresses, breathing in impacted. Clinicians follow
a person’s breathing ability, called vital capacity, using a number of
different measures such as slow vital capacity and forced vital capacity. While
different in name, these measures generally provide the clinician information
about the patient’s ability to breathe normally and in a distressed situation.
Following how these and other data change over time enable clinicians to
consider making recommendations to patients about different respiratory
interventions, including the use non-invasive ventilation devices, such as a
BiPap machine. The use of NIV devices has been shown repeatedly in research and
practice to extend the life of people with ALS. However, according to Carlayne
Jackson, M.D., chief medical officer of the University of Texas in San Antonio,
only a third of those with ALS that have a FVC reading of 50% or less use NIV.
Jackson presented original research on NIV use and
compliance in people with ALS. Jackson sought to understand if people with ALS
would be more likely to comply with NIV use if they were introduced to it
earlier on in disease. To begin to determine this, she and her colleagues
recruited 46 people into a research study in which 29 were introduced to NIV
use very early on, when their FVC was at or above 80% and compared their
compliance of use over time to the remaining cohort which received NIV
instruction when their FVC dropped below 80%, which is when Jackson reported
the intervention was more commonly introduced.
Compliance for both groups was defined as using NIV for 4
hours or more on 60% of the days it was recommended. Those introduced to NIV
earlier on in disease progression did have a greater rate of compliance.
Jackson and her colleagues hope to conduct a larger more well powered study in
the future.
For at least the last decade, the topic of exercise and ALS
has been a hot one. Plenty of research has been published regarding injuries
from sports exercise, such as concussions, and the role that that can have on
the onset of ALS. However, less attention is given to the role that exercise
may play in the treatment of ALS. Several researchers, including Annerieke C
van Groenestijn and her colleagues at Utrecht University in the Netherlands,
are seeking to understand the potential role aerobic exercise may play in the
care of individuals with ALS.
Groenestijn reported results from a 16 week long research
study of aerobic exercise at this year’s symposium. 27 people with ALS were
enrolled into the treatment group in her study. These individuals received
training three times a week in which they would exercise both using a step
board and a stationary bicycle (ergometer).
Individuals in the treatment group would “train” twice a week at home
and once a week at the clinic, whereas another 30 individuals recruited in the
study received no training. The study lasted 10 months in total, with a 6 month
follow up period to determine if the exercise routine had any impact on
survival.
Only 18 individuals recruited into the treatment arm of the
program completed the entire 10 months of training. The other nine individuals
decided to continue their own exercise routines rather than those being
studied. Groenestijn’s analysis of the data showed the exercise routine was
likely too burdensome for most. However, the data did show a positive trend on
survival in those diagnosed with ALS who had a seemingly slower disease
progression and were already exercising at a higher than average rate when they
were first diagnosed with ALS. Groenestijn and her colleagues concluded that
other studies are needed to determine the potential role exercise may have on
the progression of disease, including which exercises may have the greatest
impact overall.
First Clinical
Trial of Cannabis in People with ALS Reported
Gabriele Mora, Ph.D., reported on the first ever randomized
study of cannabis on the impact of disease progression in people with ALS.
Specifically, Mora and colleagues in Italy sought to investigate the impact of
an oral formulation of cannabis in people with either ALS or PLS. The study
began to enroll individuals in 2014 and results from the recently completed
analysis were presented.
With many anecdotal reports coming from people with ALS
regarding the use of cannabis on their quality of life, Mora and colleagues
decided to look at pain, ALSFRS-R, impression, appetite, weight and safety,
among other things, in their study. A total 60 individuals were successfully
recruited, with 30 of them being randomized into the treatment group and the
other half into the placebo group. This double blind arm of the study lasted
four weeks, and then all participants were invited to participate in a four
week open label arm of the study.
Mora reported that the most statistically significant impact
found was on spasticity, as self-reported by participants using the Ashworth
scale. While the treatment did prove to be safe and well tolerated, no other
measures reached statistical significance. A larger study, perhaps on different
forms of cannabis formulations, ought to be conducted to learn more about its
potential impact on the progression of ALS, according to Mora.
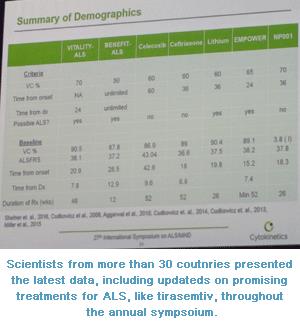 VITALITY-ALS
(tirasemtiv) Clinical Trial Completes Enrollment, Initial Demographics Released
VITALITY-ALS
(tirasemtiv) Clinical Trial Completes Enrollment, Initial Demographics Released
For more than a decade now, Cytokinetics, Inc. has been
advancing its fast-twitch skeletal muscle troponin activator, tirasemtiv,
through clinical trials in ALS. Well over 1000 people have now been treated
with this compound through previous phase 2 clinical trials and the current
multi-national phase 3 clinical trial known as VITALITY-ALS. Previous research
has provided researchers at Cytokinetics with important information regarding
how to best dose tirasemtiv as well as to how best to measure its impact on
people with ALS. The current, pivotal phase 3 clinical trial opened for
enrollment in September 2015 and completed enrollment of more than 600 people
with ALS worldwide in August 2016. This makes the study one of the largest and
most rapidly enrolling studies in ALS history.
The chair of the Department of Neurology at Barrow
Neurological Institute in Phoenix, AZ, Jeremy Shefner, M.D., Ph.D., is a
principal investigator of VITALITY-ALS for Cytokinetics, and provided an update
on the pivotal phase 3 study at this year’s international symposium. He
reported that on average, enrollees were 20 months from their first symptoms of
ALS when they started in the research study. At time of enrollment, study
participants had a mean ALSFRS-R score of 38 (out of a possible 48) and a mean
vital capacity score of 90%. The phase 3 study has total impact on vital
capacity and ALSFRS-R as outcome measures.
Earlier studies had shown potential for adverse events in
some people with ALS, so all enrolled in VITALITY-ALS were given the active
compound for two weeks rather than the one week open label lead in phase used
in the previous BENEFIT-ALS phase 2 study. Shefner reported that this longer
open label lead in phase of the trial found a similar percentage (20%) of
individuals unable to tolerate the lowest dose of the compound as in the
earlier study.
Shefner and the Cytokinetics team expects the study to be
completed in August 2017 and for the team at Cytokinetics to be able to read
out the final results before the international symposium gathers again in December
in Boston next year. The results of this clinical trial will be among the most
anticipated in recent years.
Edaravone Phase 3
Study from Japan Results Presented and Discussed
Last year, the Japanese Pharmaceutical Agency approved the
marketing of Radicut® as a treatment for ALS in that country. This approval
came after nearly a decade of research of the free-radical scavenger in people
with ALS in Japan. Edaravone is delivered via intravenous infusion to people
with ALS either once or twice daily, depending on the severity of their disease
and in accordance with their physician’s prescription. Joseph Palumbo, M.D.,
Ph.D., Chief Medical Officer of Mitsubishi Tanabe Development Corporation took
to the dais in Dublin to give one of the most awaited presentations of the week
regarding the open label extension arm of a phase 3 study conducted in Japan.
Palumbo walked through the history of edaravone (aka
Radicut, MCI-186, etc) moving forward over the past decade, including the
results from the first phase 3 study of the compound, which failed to meet its
endpoints. It was only after the researchers conducted a post-hoc analysis of
that study that they found what they considered to be a responder group.
Included in this group were those who had first symptoms of disease within the
past 20 months, received a probable or definitive ALS diagnosis via the El
Escorial criteria and have at least two points on every question on the
ALSFRS-R at time of enrollment.
The company decided to execute a follow up phase 3 clinical
trial enrolling only individuals who met this responder group criteria and
Palumbo presented some of the data from that study. A total of 137 people with
ALS enrolled in that study, 69 of which received edaravone and 68 received
placebo for 24 weeks, and then all were offered to continue in an open-label
extension of the study. Those treated with edaravone in this phase 3 study had a slowing of disease progression occur. This slowing, as measured using the ALSFRS-R scale, was statistically significant. Japan's regulatory agency approved the marketing of Radicut as a treatment for ALS based on these results in 2015. Further reporting at the ALS
symposium by Wendy Agense of Mitsubishi Tanabe showed that there were also
trends towards a slowing of progression using the King’s and MITOS Clinical
Stages Systems.
Mitsubishi Tanabe Pharma America, Inc. has filed a New Drug
Application for edaravone (to be marketed as Radicava®) with the Food &
Drug Administration in the United States of America and expects a decision by
the summer of 2017.
NurOwn® Clinical
Trial Results Presented as Late Breaking News
The symposium ended with a presentation added regarding
results from a phase 2 clinical trial conducted by BrainStorm Cell Therapeutics
on their product NurOwn® in people with ALS. NurOwn is a cell therapy using a
person’s own mesenchymal stem cells, which are harvested and modified to become
glial progenitor cells designed to express neurotrophic factors (NTF). The
cells are then transplanted back into the original donor during a single round
of intramuscular and intraspinal infusions.
James Berry, M.D., MPH, head of the ALS Clinic at
Massachusetts General Hospital and a principal investigator of the study,
presented the findings. He reported that a total of 46 individuals with ALS
participated, with 35 receiving treatment and 11 receiving placebo infusions.
Berry reported the procedures to be both safe and well
tolerated, although he reported that some individual did experience serious
adverse events (i.e. the need for a feeding tube) following treatment, although
the relationship between the treatment and those SAEs weren’t likely connected,
rather simply related to the eventual progression of the disease.
Impact on ALSFRS-R scores were also reviewed by Berry,
showing effects on the proposed responder group of individuals to be enrolled
in the study. This responder group, determined by BrainStorm in advance, was
designed to look at fast progressors vs. slow progressions by looking at rate
of progression during the three month lead in period on ALSFRS-R and vital
capacity.
He also reported initial results showing an increase in NTF
expression in cerebrospinal fluid harvested from individuals before and after
treatment with NurOwn. Berry reported that a larger clinical research study is
needed in which multiple doses of NurOwn can be explored in people with
ALS. A few days after the symposium,
BrainStorm Cell Therapeutics announced that it has outlined a phase 3 trial
design with the FDA and expects to begin enrollment in the second quarter of
2017. That trial will include people with ALS in the United States as well as
Israel.
 Note from the author:
I have no conflicts of interest to report with any of the companies on which I
have reported on above. However, I was elected a Board Member of the
International Alliance of ALS/MND Associations during this year’s board meeting
of that organization which occurred just prior to the symposium. You can read a
recap of that meeting here. I also serve as a member of the Program Committee
of the Allied Health Professionals Forum. I am not financially compensated for
either of these voluntary positions. I was proud to represent the ALS Therapy Development Institute together with Kyle, Michele and Steve. Sláinte!
Note from the author:
I have no conflicts of interest to report with any of the companies on which I
have reported on above. However, I was elected a Board Member of the
International Alliance of ALS/MND Associations during this year’s board meeting
of that organization which occurred just prior to the symposium. You can read a
recap of that meeting here. I also serve as a member of the Program Committee
of the Allied Health Professionals Forum. I am not financially compensated for
either of these voluntary positions. I was proud to represent the ALS Therapy Development Institute together with Kyle, Michele and Steve. Sláinte!